Calcium phosphate cement injectable for regeneration of alveolar bone contains gentamycin
TCP cement helps in maintaining bony defect spaces and permits bone growth on their surface or into channels, pores, or pipes, and primarily acts as osteoconductive materials. The osteoinductive and osteoconductive features of calcium phosphates are also important for bone regeneration. Osteoinduction is the ability to induce progenitor cells to differentiate into osteoblastic lineages , whereas osteoconduction is the ability of bone growth on the surface of materials .
The direct way to new bone
Today, bone regeneration and replacements materials are used in dentistry, orthopaedics to treat traumatic or diseased bone defects.
Ossmpore is a restorable ,synthetic biocompatible sterile Beta TCP material, which has capacity to replace bone and subsequently induce bone deposition. it initially acts as filler and scaffold for regeneration of bone ..
Safety
Osspore is free from immunological risks or risk of infections connected with materials of biological origin. osspore completely resorbed while inducing formation of autologous bone. our strict quality control ystems ensure osspore are made to highest standard. infact the synthesis of osspore made to internationally recognized norm ASTM F1088-04 (Standard Specification for ß-Tricalciumphosphate for Surgical Implantation) require purity of TCP to be above 95 however, our is 99%
OSSPORE CP
- A calcium hydroxyapitite, Calcium Phosphate and calcium Sulphate ( complex calcium Phosphate) a chemical and
structural similarity to the mineral phase of bone
Features
- Osteoconductive, which means it forms a bond with osteoblasts that form new bone
- Good flow and
40 wt% hydroxyapatite (HA),10% TCP :48 ww%calcium sulfate hemihydrate
(As calcium Phosphate complex)
Description
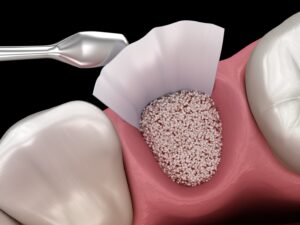
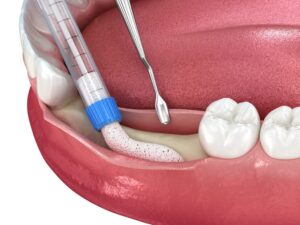
OSSPORE CP BONE VOID FILLER is a fast setting, injectable and mouldable synthetic bone graft substitute material intended for bone voids/gaps.
The material consists of a powder and a liquid components. The major constituents of the powder are hydroxy apatite/phosphate and calcium sulfate hemihydrate and TCP. .
The ceramic bone substitute material is placed into the bone void under visual inspection or under radiographic monitoring during open or percutaneous surgery.
The paste may be injected into the defect, moulded by hand and digitally placed into the defect, or used to prepare beads that are placed into the defect. When fully set in vivo,
Contraindications
- Hypersensitivity to any of the excipients
- Local infection at the site of implantation
- Pregnancy
- Breastfeeding
- Manifest thyrotoxicosi
OSSPORE CP MATERIAL SPECIFICATIONS:
Setting temperature: MAX TEMP<43°C
Initial compressive: 10–12 MPa (wet conditions) 65–75 MPa (dry conditions),
Final compressive Strength :20–40 % Average pore size 1 micron
Handling Injectable
Mouldable (by hand) – for up to 1 minute max.
Also for use with a bead mould tray (not included in pack) Drillable
Compatibility with Autograft, allograft, hardware
SETTING PARAMETERS
Pre mix Liquid and powder and and fill syringe: 2min Inject: 2min
Wait for paste to set 10min Drill after 15min
Mixing and Moulding by hand
Mix powder and liquid: 2-3 minwait for 5min Start moulding : 1min
Place in void and sculptor for upto 3min Allow to set: 3min
Drill final set for attachments. Bead tray use.
Mix and fill tray: 5min wait to harden:10mins
Release from tray and place in defect.
Use press mix material to seal in edges and overlap host bone. Volume
PRESENTATION
OSSPORE CP G (GENTAMYCIN) OSSPORE CP V (VANCOPASTE
Order code MEDICAL
5mL CP105
10mL CP110
8mL Cp118
DENTAL
1ML CP100
ifu
DCL OSSPORE CP
• A calcium hydroxyapitite, Calcium Phosphate and calcium Sulphate ( complex calcium Phosphate) a chemical and
structural similarity to the mineral phase of bone
Features
• Osteoconductive, which means it forms a bond with osteoblasts that form new bone
• Good flow and injectable.
40 wt% hydroxyapatite (HA),10% TCP :48 ww%calcium sulfate hemihydrate
(As calcium Phosphate complex)
Description
OSSPORE CP BONE VOID FILLER is a fast setting, injectable and mouldable synthetic bone graft substitute material intended for bone voids/gaps.
The material consists of a powder and a liquid components. The major constituents of the powder are hydroxy apatite/phosphate and calcium sulfate hemihydrate and TCP. .
The ceramic bone substitute material is placed into the bone void under visual inspection or under radiographic monitoring during open or percutaneous surgery.
The paste may be injected into the defect, moulded by hand and digitally placed into the defect, or used to prepare beads that are placed into the defect. When fully set in vivo,
Contraindications
• Hypersensitivity to any of the excipients
• Local infection at the site of implantation
• Pregnancy
• Breastfeeding
• Manifest thyrotoxicosi
OSSPORE CP MATERIAL
SPECIFICATIONS:
Setting temperature: MAX TEMP<43°C
Initial compressive: 10–12 MPa (wet conditions)
65–75 MPa (dry conditions),
Final compressive Strength :20–40 %
Average pore size 1 micron
Handling
Injectable
Mouldable (by hand) – for up to 1 minute max.
Also for use with a bead mould tray (not included in pack)
Drillable
Compatibility with Autograft, allograft, hardware
SETTING PARAMETERS
Pre mix Liquid and powder and and fill syringe: 2min
Inject: 2min
Wait for paste to set 10min
Drill after 15min
Mixing and Moulding by hand
Mix powder and liquid: 2-3 minwait for 5min
Start moulding : 1min
Place in void and sculptor for upto 3min
Allow to set: 3min
Drill final set for attachments.
Bead tray use.
Mix and fill tray: 5min
wait to harden:10mins
Release from tray and place in defect.
Use press mix material to seal in edges and overlap host bone.
Volume
PRESENTATION
OSSPORE CP G (GENTAMYCIN)
OSSPORE CP V (VANCOPASTE
Order code
MEDICAL
5mL CP105
10mL CP110
8mL Cp118
DENTAL
1ML CP100